Limitations of PCR Only
PCR testing for UTI’s may not tell the whole story.
Polymerase Chain Reaction (PCR) testing is often utilized to identify the organisms found when Urinary Tract Infection (UTI) testing is performed. Results from PCR testing are intended to support the practitioner’s ability to order the most effective antibiotic and determine infections that might have been missed with traditional urine tests. However, when performing PCR only testing, how do you know if the organism detected is viable, and therefore part of what is causing infection, and that the resistance genes are expressed and causing antibiotic resistance? Treatment based on the results could potentially lead to overtreatment and unnecessary antibiotic use.
PCR testing uses amplification of targeted genetic material to allow for low concentrations to be detected. It is widely used in laboratories in the identification of pathogens for the diagnosis of infectious diseases. Since PCR amplifies genetic material, it can detect both live and nonviable organisms. Although promising, research studies have not yet shown a significant association between UTI PCR results alone and improved outcomes.
PCR is more specific than traditional methods which can help identify uropathogens present that are difficult to detect with culture, and therefore, provide information to help with treatment selection. Molecular diagnostic technologies such as multiplex PCR offer high sensitivity, specificity, and accuracy in identifying several bacterial targets in the same reaction.1 Guidance® UTI has demonstrated 95% sensitivity when identifying organisms and has been shown to detect bacteria in 43% more cases than standard urine culture.1
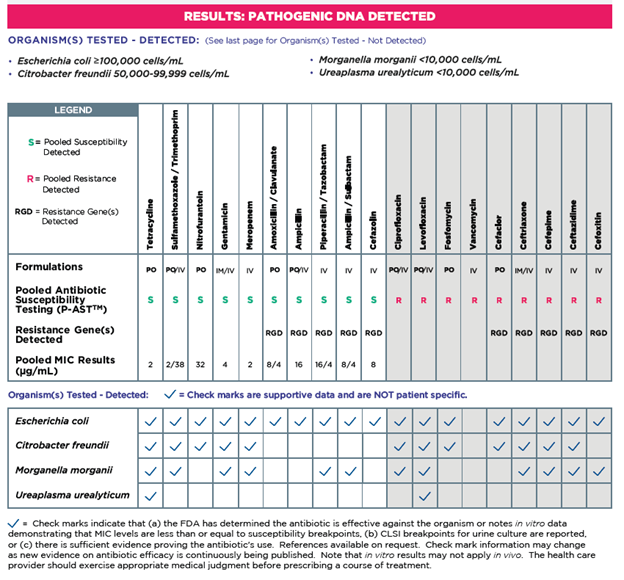
Resistance Genes Detected (RGD) does not equal susceptibility testing. On average, genotypic results disagree with phenotypic (observed) resistance 40% of the time on average.2 Sometimes antibiotic resistant genes are not fully expressed and could be impacted by mutations or changes in resistance due to interactions with other organisms. Guidance® UTI combines genotypic and phenotypic P-AST results, allowing for more informed treatment decisions.
PCR alone does not perform susceptibility testing. Guidance® UTI provides more information to prescribe personalized therapy options for each individual patient. On the report, checkmarks are present if the antibiotic has supporting evidence or FDA approval for use with the organism. Guidance® UTI provides the sensitivity of PCR for organism identification and antibiotic resistance gene detection with a unique technology called Pooled Antibiotic Susceptibility Testing (P-AST™).
Let’s review the data. Guidance® UTI has a large body of evidence, including 3 clinical studies with over 68,000 subjects and 6 peer-reviewed publications. Recent real-world evidence presented at AUA shows that Guidance® UTI testing is associated with reductions in critical adverse outcomes, healthcare resource utilization and cost for complicated UTI, including 13% lower rate of outpatient emergency visits, 67% lower inpatient admission rate, and $463.46 saving in medical resource utilization per complicated UTI (cUTI) patient tested with Guidance® UTI.3
Not all UTI tests are the same! To learn more about Guidance® UTI click here.
- Vollstedt A, Baunoch D, Wojno KJ, Luke N, Cline K, et al. (2020) Multisite Prospective Comparison of Multiplex Polymerase Chain Reaction Testing with Urine Culture for Diagnosis of Urinary Tract Infections in Symptomatic Patients. J Sur urology: JSU-102.
- Baunoch D, Luke N, Wang D, Vollstedt A, Zhao X, Ko DSC, Huang S, Cacdac P, Sirls LT. Concordance Between Antibiotic Resistance Genes and Susceptibility in Symptomatic Urinary Tract Infections. Infect Drug Resist. 2021 Aug 19;14:3275-3286
- Aparna Ashok, Dicken Ko, Providence RI, Emily Lukacz, La Jolla, CA, Annah Vollstedt, Iowa City, IA, Iver Juster, San Rafael, CA, Timothy Niecko, Tierra Verde, FL, David Baunoch, Trabuco Canyon, CA, Mohit Mathur, Irvine, CA. Comparison of Guidance® UTI and standard urine culture for rates of sepsis, hospitalization and other adverse outcomes in complicated urinary tract infections [abstract]. Journal of Urology. AUA Annual Meeting Program Abstracts 2022. 1 May 2022. Volume 207 Issue Supplement 5 May 2022