Ampicillin-Sulbactam
Emery Haley, PhD, Scientific Writing Specialist
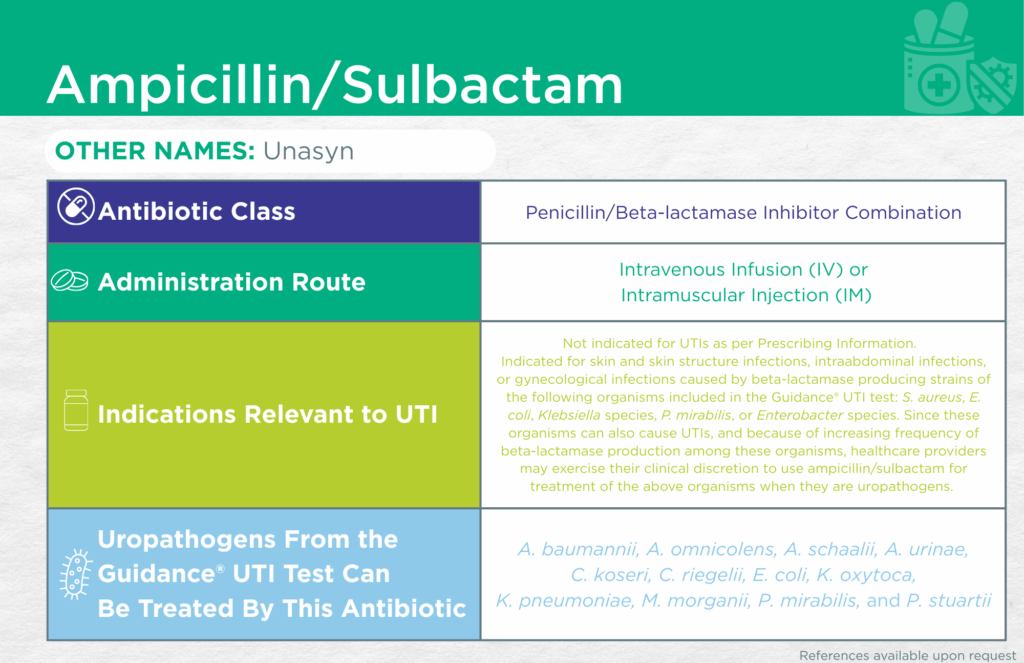
Ampicillin/Sulbactam
Find the Latest FDA-Approved Labelling Information Here: Drugs@FDA Online Database
Administrative Routes
Parenteral [intravenous infusion (IV) or intramuscular injection (IM)]
Other Names
Unasyn
Bacteriostatic or Bactericidal
Bactericidal [1]
Antibiotic Class
Penicillin/Beta-lactamase Inhibitor Combination
Mechanisms of Action
This antibiotic leverages dual mechanisms of action.
Ampicillin, a beta-lactam antibiotic, binds to penicillin-binding proteins (PBPs) on bacterial cell walls. PBPs are essential for the formation of the peptidoglycan, which gives the bacterial wall strength and integrity. Binding of ampicillin to PBPs leads to failure of peptidoglycan cell wall synthesis, causing bacterial cell death.
Some bacteria develop resistance to beta-lactam antibiotics, like ampicillin, by producing enzymes, called beta-lactamases, that degrade the antibiotic. Sulbactam helps preserve the activity of beta-lactam antibiotics by inhibiting these enzymes and allowing ampicillin to then bind to PBPs and cause bacterial cell wall degradation.
WHO AWaRe Classification
Access [2]
Empiric Use Recommendations
No published guidance
Indication(s) Relevant to UTI
Not indicated for UTIs as per Prescribing Information.
Indicated for skin and skin structure infections, intraabdominal infections, or gynecological infections caused by beta-lactamase producing strains of the following organisms included in the Guidance UTI test: Staphylococcus aureus, Escherichia coli, Klebsiella species, Proteus mirabilis, or Enterobacter species.
Since these organisms can also cause UTIs, and because of increasing frequency of beta-lactamase production among these organisms, healthcare providers may exercise their clinical discretion to use ampicillin/sulbactam for treatment of the above organisms when they are uropathogens.
Checkmarks
CLSI and/or FDA documents support the efficacy of this antibiotic against the following organisms from the Guidance® UTI test: Acinetobacter baumannii, Aerococcus urinae, Citrobacter koseri, Corynebacterium riegelii, Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Morganella morganii, Proteus mirabilis, and Providencia stuartii.
Published primary literature supports the efficacy of this antibiotic against the following organisms from the Guidance® UTI test: Alloscardovia omnicolens [3], and Actinotignum schaalii [4-6]
- Ishak, A.; Mazonakis, N.; Spernovasilis, N.; Akinosoglou, K.; Tsioutis, C. Bactericidal versus Bacteriostatic Antibacterials: Clinical Significance, Differences and Synergistic Potential in Clinical Practice. J. Antimicrob. Chemother. 2024, 80, 1–17, doi:10.1093/jac/dkae380
- AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use, 2023 Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 6 February 2025).
- Isnard, C.; Lienhard, R.; Reissier, S.; Rodriguez, S.; Krähenbühl, J.; Liassine, N.; Guérin, F.; Cattoir, V. In Vitro Antimicrobial Susceptibility of Alloscardovia Omnicolens and Molecular Mechanisms of Acquired Resistance. Diagnostic Microbiology and Infectious Disease 2016, 84, 227–229, doi:10.1016/j.diagmicrobio.2015.08.009.
- Lotte, R.; Lotte, L.; Ruimy, R. Actinotignum Schaalii (Formerly Actinobaculum Schaalii): A Newly Recognized Pathogen—Review of the Literature. Clin Microbiol Infec 2016, 22, 28–36, doi:10.1016/j.cmi.2015.10.038.
- Beguelin, C.; Genne, D.; Varca, A.; Tritten, M. ‐L.; Siegrist, H.H.; Jaton, K.; Lienhard, R. Actinobaculum Schaalii: Clinical Observation of 20 Cases. Clin Microbiol Infec 2011, 17, 1027–1031, doi:10.1111/j.1469-0691.2010.03370.x.
- Sahuquillo-Arce, J.M.; Suárez-Urquiza, P.; Hernández-Cabezas, A.; Tofan, L.; Chouman-Arcas, R.; García-Hita, M.; Sabalza-Baztán, O.; Sellés-Sánchez, A.; Lozano-Rodríguez, N.; Martí-Cuñat, J.; et al. Actinotignum Schaalii Infection: Challenges in Diagnosis and Treatment. Heliyon 2024, 10, e28589, doi:10.1016/j.heliyon.2024.e28589.
Dr. Emery Haley is a scientific writing specialist with over ten years of experience in translational cell and molecular biology. As both a former laboratory scientist and an experienced science communicator, Dr. Haley is passionate about making complex research clear, approachable, and relevant. Their work has been published in over 10 papers and focuses on bridging the gap between the lab and real-world patient care to help drive better health outcomes.