Clindamycin
Emery Haley, PhD, Scientific Writing Specialist
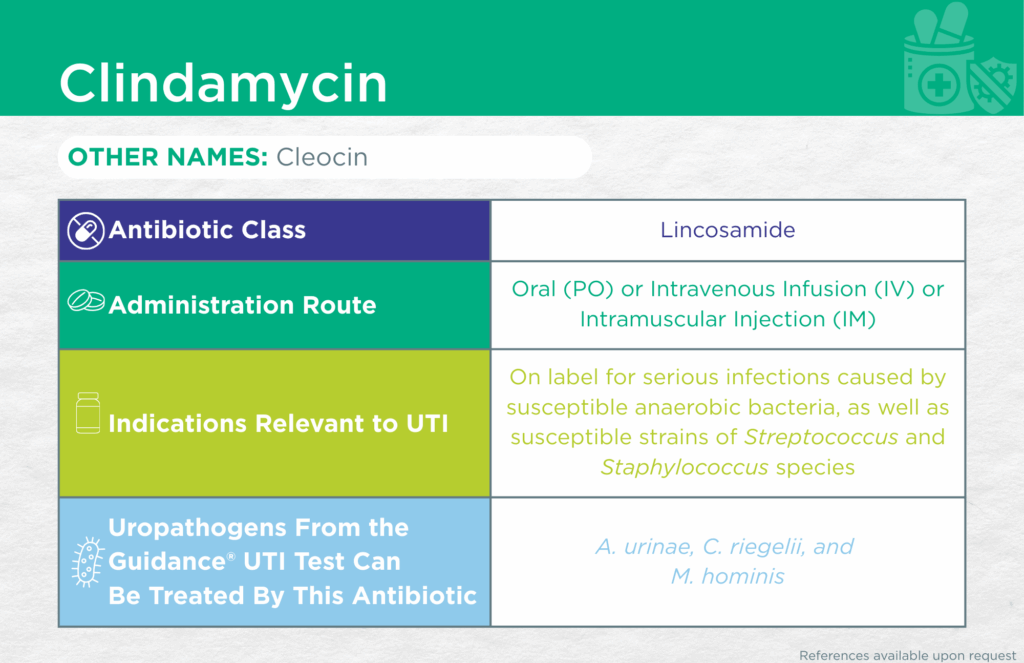
Clindamycin
Find the Latest FDA-Approved Labelling Information Here: Drugs@FDA Online Database
Administrative Routes
Oral (PO) or Parenteral [intravenous infusion (IV) or intramuscular injection (IM)]
Other Names
Cleocin
Bacteriostatic or Bactericidal
Bacteriostatic [1]
Antibiotic Class
Lincosamide
Mechanisms of Action
The drug binds to the 50S ribosomal subunit of bacterial ribosomes. Binding to bacterial ribosomes blocks bacterial cells from synthesizing proteins required for growth.
WHO AWaRe Classification
Access [2]
Empiric Use Recommendations
No published guidance
Indication(s) Relevant to UTI
On label for serious infections caused by susceptible anaerobic bacteria, as well as susceptible strains of Streptococcus and Staphylococcus species.
Checkmarks
CLSI and/or FDA documents support the efficacy of this antibiotic against the following organisms from the Guidance® UTI test: Aerococcus urinae, Corynebacterium riegelii, and Mycoplasma hominis.
- Ishak, A.; Mazonakis, N.; Spernovasilis, N.; Akinosoglou, K.; Tsioutis, C. Bactericidal versus Bacteriostatic Antibacterials: Clinical Significance, Differences and Synergistic Potential in Clinical Practice. J. Antimicrob. Chemother. 2024, 80, 1–17, doi:10.1093/jac/dkae380
- AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use, 2023 Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 6 February 2025).
Dr. Emery Haley is a scientific writing specialist with over ten years of experience in translational cell and molecular biology. As both a former laboratory scientist and an experienced science communicator, Dr. Haley is passionate about making complex research clear, approachable, and relevant. Their work has been published in over 10 papers and focuses on bridging the gap between the lab and real-world patient care to help drive better health outcomes.