E. faecium
Emery Haley, PhD, Scientific Writing Specialist
Enterococcus faecium
Clinical Summary
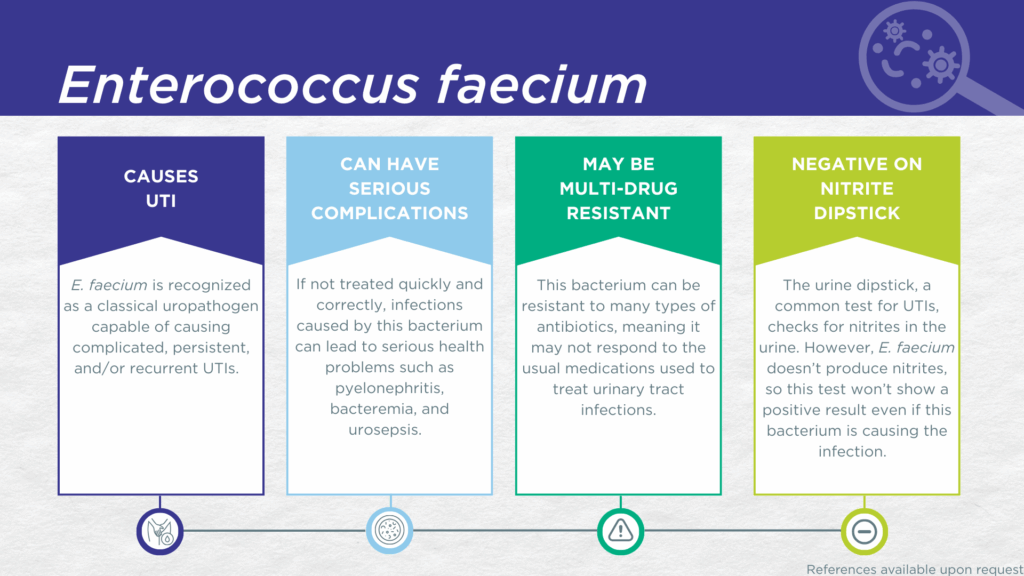
- E. faecium is recognized as a classical, gram-positive, biofilm-forming, nitrite-negative uropathogen.
- E. faecium is primarily associated with highly antibiotic-resistant catheter-associated UTIs (CAUTIs), hospital-acquired UTIs (HAUTIs), and complicated UTIs among immunocompromised individuals and individuals with urinary tract abnormalities.
- In symptomatic UTI patients, E. faecium:
- Is not a contaminant (is found in catheter-collected urine specimens).
- Is viable (can grow out on culture).
- Is pathogenic (associated with elevated urine biomarkers of infection).
- Reported severe complications of E. faecium UTI include pyelonephritis, bacteremia, urosepsis, and death.
- Multidrug-resistant E. faecium is a significant and well-studied global health threat.
Bacterial Characteristics
Gram-stain
Gram-positive
Morphology
Coccus
Growth Requirements
Non-fastidious (grows moderately well in standard urine culture conditions)
Facultative anaerobe/microaerophile
Nitrate Reduction
No
Urease
Negative
Biofilm Formation
Yes
Pathogenicity
Colonizer or Pathobiont
Clinical Relevance in UTI
E. faecium is a gram-positive, biofilm-forming microorganism classically recognized as a uropathogen. E. faecium is the second most commonly reported gram-positive uropathogen (after E. faecalis).[1] However, E. faecium lacks nitrate reductase activity, so screening strategies involving urinalysis for nitrite positivity will be false-negative.[2,3]
E. faecium is known for facilitating hard-to-treat polymicrobial infections involving multidrug resistance and biofilms.[4] In preclinical studies of polymicrobial UTI, E. faecium exhibited synergism with P. aeruginosa.[5] E. faecium is primarily detected in complicated UTIs, especially among immunocompromised individuals and individuals with urinary tract abnormalities or indwelling catheters.[6,7] Most reported E. faecium UTIs are catheter-associated UTIs (CAUTIs) and/or healthcare-acquired UTIs (HAUTIs).[7–10] Compared to E. faecalis, E. faecium UTIs are less common but are significantly more likely to be multidrug or pandrug resistant.[7–10]
In a study of older adult males and females with clinically suspected complicated UTI, E. faecium was detected in both midstream voided and in-and-out-catheter collected specimens indicating that it was truly present in the bladder, not simply a contaminant picked up during voiding.[11] Furthermore, elevated markers of immune system activation in the urinary tract have been measured from the same clinical urine specimens in which E. faecium was detected, indicating that the presence of E. faecium was associated with an immune response to urinary tract infection.[12–14]
E. faecium is one of the six so-called “ESKAPE pathogens” identified as critical multi-drug resistant bacteria requiring urgent development of effective therapeutics.[15] Severe reported complications of E. faecium UTI include pyelonephritis, bacteremia, urosepsis, and death.[16–18]
Together, these findings indicate that E. faecium should be seriously considered as a uropathogen and demonstrate the value of detecting this organism in any individual with an indwelling catheter, urinary tract abnormality, immunocompromise, or other risk factors for complicated UTI.
Treatment
Evidence of Efficacy (Checkmarks): Ciprofloxacin, Levofloxacin, Linezolid, Nitrofurantoin, and Vancomycin.
1. Khalil, M.A.; Alorabi, J.A.; Al-Otaibi, L.M.; Ali, S.S.; Elsilk, S.E. Antibiotic Resistance and Biofilm Formation in Enterococcus Spp. Isolated from Urinary Tract Infections. Pathogens 2022, 12, 34, doi:10.3390/pathogens12010034.
2. BacDive | The Bacterial Diversity Metadatabase Available online: https://bacdive.dsmz.de/ (accessed on 11 February 2025).
3. BioCyc Pathway/Genome Database Collection Available online: https://biocyc.org/ (accessed on 11 February 2025).
4. Xu, W.; Fang, Y.; Zhu, K. Enterococci Facilitate Polymicrobial Infections. Trends Microbiol. 2024, 32, 162–177, doi:10.1016/j.tim.2023.07.010.
5. Laliany, G.; Jamehdar, S.A.; Makhdoumi, A. In Vitro Virulence Activity of Pseudomonas Aeruginosa, Enhanced by Either Acinetobacter Baumannii or Enterococcus Faecium through the Polymicrobial Interactions. Arch. Microbiol. 2022, 204, 709, doi:10.1007/s00203-022-03308-8.
6. Coman, F.; Codău, C.-A.; Novac, B. Breaking the Stone and Beating the Bug: Managing Ureterolithiasis with Enterococcus Faecium in a High-Risk Patient. Arch. Clin. Cases 2025, 12, 51–53, doi:10.22551/2025.46.1201.10312.
7. Eure, T.R.; Stone, N.D.; Mungai, E.A.; Bell, J.M.; Thompson, N.D. Antibiotic-Resistant Pathogens Associated with Urinary Tract Infections in Nursing Homes: Summary of Data Reported to the National Healthcare Safety Network Long-Term Care Facility Component, 2013–2017. Infect. Control Hosp. Epidemiology 2021, 42, 31–36, doi:10.1017/ice.2020.348.
8. Kraszewska, Z.; Skowron, K.; Kwiecińska-Piróg, J.; Grudlewska-Buda, K.; Przekwas, J.; Wiktorczyk-Kapischke, N.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Antibiotic Resistance of Enterococcus Spp. Isolated from the Urine of Patients Hospitalized in the University Hospital in North-Central Poland, 2016–2021. Antibiotics 2022, 11, 1749, doi:10.3390/antibiotics11121749.
9. Georges, M.; Odoyo, E.; Matano, D.; Tiria, F.; Kyany’a, C.; Mbwika, D.; Mutai, W.C.; Musila, L. Determination of Enterococcus Faecalis and Enterococcus Faecium Antimicrobial Resistance and Virulence Factors and Their Association with Clinical and Demographic Factors in Kenya. J. Pathog. 2022, 2022, 3129439, doi:10.1155/2022/3129439.
10. Ahmed, J.; Yadav, R.K.; Sood, S.; Das, B.K.; Dhawan, B. Vancomycin-Resistant Enterococcus Faecium: A High Priority Pathogen. J. Appl. Sci. Clin. Pr. 2023, 4, 168–176, doi:10.4103/jascp.jascp_17_23.
11. Wang, D.; Haley, E.; Luke, N.; Mathur, M.; Festa, R.; Zhao, X.; Anderson, L.A.; Allison, J.L.; Stebbins, K.L.; Diaz, M.J.; et al. Emerging and Fastidious Uropathogens Were Detected by M-PCR with Similar Prevalence and Cell Density in Catheter and Midstream Voided Urine Indicating the Importance of These Microbes in Causing UTIs. Infect. Drug Resist. 2023, Volume 16, 7775–7795, doi:10.2147/idr.s429990.
12. Haley, E.; Luke, N.; Mathur, M.; Festa, R.A.; Wang, J.; Jiang, Y.; Anderson, L.A.; Baunoch, D. The Prevalence and Association of Different Uropathogens Detected by M-PCR with Infection-Associated Urine Biomarkers in Urinary Tract Infections. Res. Rep. Urol. 2024, 16, 19–29, doi:10.2147/rru.s443361.
13. Akhlaghpour, M.; Haley, E.; Parnell, L.; Luke, N.; Mathur, M.; Festa, R.A.; Percaccio, M.; Magallon, J.; Remedios-Chan, M.; Rosas, A.; et al. Urine Biomarkers Individually and as a Consensus Model Show High Sensitivity and Specificity for Detecting UTIs. BMC Infect Dis 2024, 24, 153, doi:10.1186/s12879-024-09044-2.
14. Parnell, L.K.D.; Luke, N.; Mathur, M.; Festa, R.A.; Haley, E.; Wang, J.; Jiang, Y.; Anderson, L.; Baunoch, D. Elevated UTI Biomarkers in Symptomatic Patients with Urine Microbial Densities of 10,000 CFU/ML Indicate a Lower Threshold for Diagnosing UTIs. MDPI 2023, 13, 1–15, doi:10.3390/diagnostics13162688.
15. Miller, W.R.; Arias, C.A. ESKAPE Pathogens: Antimicrobial Resistance, Epidemiology, Clinical Impact and Therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616, doi:10.1038/s41579-024-01054-w.
16. Wu, Y.; Li, P.; Huang, Z.; Liu, J.; Yang, B.; Zhou, W.; Duan, F.; Wang, G.; Li, J. Four-Year Variation in Pathogen Distribution and Antimicrobial Susceptibility of Urosepsis: A Single-Center Retrospective Analysis. Ther. Adv. Infect. Dis. 2024, 11, 20499361241248056, doi:10.1177/20499361241248058.
17. Parente, G.; Gargano, T.; Pavia, S.; Cordola, C.; Vastano, M.; Baccelli, F.; Gallotta, G.; Bruni, L.; Corvaglia, A.; Lima, M. Pyelonephritis in Pediatric Uropathic Patients: Differences from Community-Acquired Ones and Therapeutic Protocol Considerations. A 10-Year Single-Center Retrospective Study. Children 2021, 8, 436, doi:10.3390/children8060436.
18. Shahab, K.; Mufti, A.Z.; Iqbal, M.A.; Roghani, M.; Zeb, F.; Amin, U. Assessment of Microbial Diversity Pattern of Sensitivity and Antimicrobial Susceptibility in Patients Admitted with Urosepsis. Ann. PIMS-Shaheed Zulfiqar Ali Bhutto Méd. Univ. 2023, 19, 339–345, doi:10.48036/apims.v19i3.924.
Dr. Emery Haley is a scientific writing specialist with over ten years of experience in translational cell and molecular biology. As both a former laboratory scientist and an experienced science communicator, Dr. Haley is passionate about making complex research clear, approachable, and relevant. Their work has been published in over 10 papers and focuses on bridging the gap between the lab and real-world patient care to help drive better health outcomes.