Gentamicin
Emery Haley, PhD, Scientific Writing Specialist
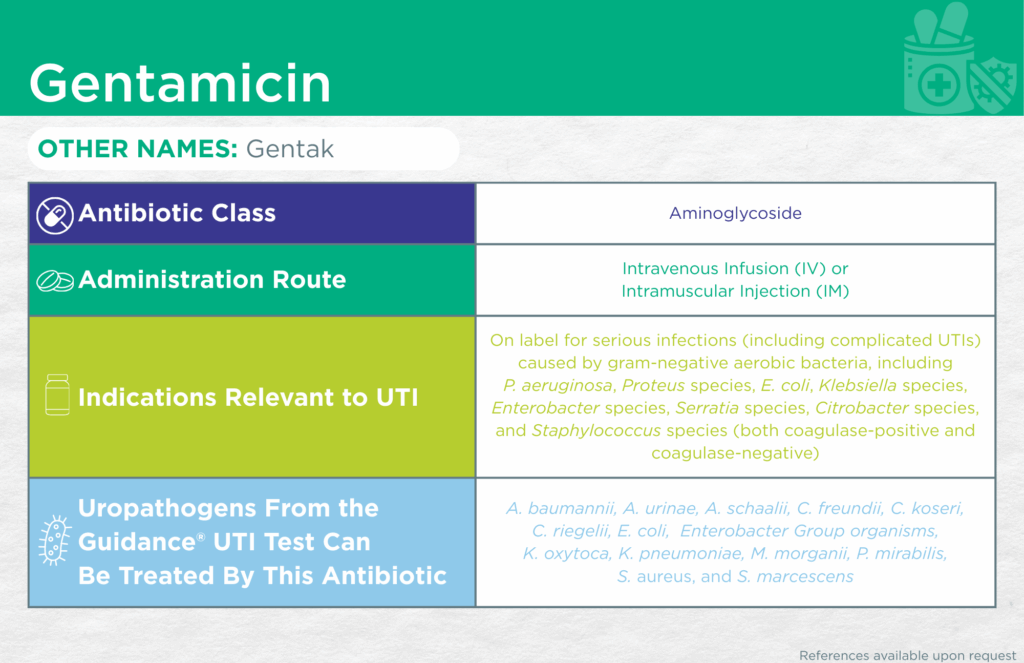
Gentamicin
Find the Latest FDA-Approved Labelling Information Here: Drugs@FDA Online Database
Administrative Routes
Parenteral [intravenous infusion (IV) or intramuscular injection (IM)]
Other Names
Gentak
Bacteriostatic or Bactericidal
Bactericidal [1]
Antibiotic Class
Aminoglycoside
Mechanisms of Action
The drug binds to the 30S ribosomal subunit of bacterial ribosomes. Binding to bacterial ribosomes blocks bacterial cells from synthesizing proteins required for growth.
WHO AWaRe Classification
Access [2]
Empiric Use Recommendations
Yes (upper UTI/pyelonephritis) [World Health Organization (WHO)] [3]
Yes (alternative for complicated UTI with or without sepsis) [Infectious Diseases Society of America (IDSA)] [4]
Indication(s) Relevant to UTI
On label for serious infections (including complicated UTIs) caused by gram-negative aerobic bacteria including Pseudomonas aeruginosa, Proteus species, Escherichia coli, Klebsiella species, Enterobacter species, Serratia species, Citrobacter species, and Staphylococcus species (both coagulase-positive and coagulase-negative).
Checkmarks
CLSI and/or FDA documents support the efficacy of this antibiotic against the following organisms from the Guidance® UTI test: Acinetobacter baumannii, Aerococcus urinae, Citrobacter freundii, Citrobacter koseri, Corynebacterium riegelii, Escherichia coli, Enterobacter Group organisms, Klebsiella oxytoca, Klebsiella pneumoniae, Morganella morganii, Proteus mirabilis, Serratia marcescens, and Staphylococcus aureus.
Published primary literature supports the efficacy of this antibiotic against the following organism from the Guidance® UTI test: Actinotignum schaalii [5-7]
- Ishak, A.; Mazonakis, N.; Spernovasilis, N.; Akinosoglou, K.; Tsioutis, C. Bactericidal versus Bacteriostatic Antibacterials: Clinical Significance, Differences and Synergistic Potential in Clinical Practice. J. Antimicrob. Chemother. 2024, 80, 1–17, doi:10.1093/jac/dkae380
- AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use, 2023 Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 6 February 2025).
- The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book – Infographics – PAHO/WHO | Pan American Health Organization Available online: https://www.paho.org/en/documents/who-aware-access-watch-reserve-antibiotic-book-infographics (accessed on 22 July 2025).
- Complicated Urinary Tract Infections (CUTI): Clinical Guidelines for Treatment and Management Available online: https://www.idsociety.org/practice-guideline/complicated-urinary-tract-infections/ (accessed on 28 July 2025).
- Lotte, R.; Lotte, L.; Ruimy, R. Actinotignum Schaalii (Formerly Actinobaculum Schaalii): A Newly Recognized Pathogen—Review of the Literature. Clin Microbiol Infec 2016, 22, 28–36, doi:10.1016/j.cmi.2015.10.038.
- Cattoir, V.; Varca, A.; Greub, G.; Prod’hom, G.; Legrand, P.; Lienhard, R. In Vitro Susceptibility of Actinobaculum Schaalii to 12 Antimicrobial Agents and Molecular Analysis of Fluoroquinolone Resistance. J Antimicrob Chemoth 2010, 65, 2514–2517, doi:10.1093/jac/dkq383.
- Sahuquillo-Arce, J.M.; Suárez-Urquiza, P.; Hernández-Cabezas, A.; Tofan, L.; Chouman-Arcas, R.; García-Hita, M.; Sabalza-Baztán, O.; Sellés-Sánchez, A.; Lozano-Rodríguez, N.; Martí-Cuñat, J.; et al. Actinotignum Schaalii Infection: Challenges in Diagnosis and Treatment. Heliyon 2024, 10, e28589, doi:10.1016/j.heliyon.2024.e28589.
Dr. Emery Haley is a scientific writing specialist with over ten years of experience in translational cell and molecular biology. As both a former laboratory scientist and an experienced science communicator, Dr. Haley is passionate about making complex research clear, approachable, and relevant. Their work has been published in over 10 papers and focuses on bridging the gap between the lab and real-world patient care to help drive better health outcomes.