Sensitivity Testing Methods for Polymicrobial Infections are NOT Created Equally
Do you value having antibiotic sensitivity testing results when deciding on treatment options for your recurrent or complicated UTI patients? You know the importance of understanding both phenotypic and genotypic information in these infections, which will help you make more informed treatment decisions and improve patient outcomes. You may also have grown frustrated with getting mixed flora or contaminated results when ordering traditional sensitivity testing and tried one of the newer advanced UTI tests that provide phenotypic results for polymicrobial infections.
But did you know there are different methods for performing antibiotic sensitivity testing? One method, called disk diffusion (or Kirby-Bauer), involves creating a “lawn” of bacteria on an agar plate and adding multiple paper disks soaked in an antibiotic (figure 1). Each disk releases the antibiotic to the surrounding area on the agar plate growing bacteria. If the antibiotic kills the bacteria or stops its growth, a clear ring will appear around the disc called the zone of inhibition. If the antibiotic disc produces a clear zone of inhibition on the plate, the bacteria is deemed sensitive to the antibiotic.
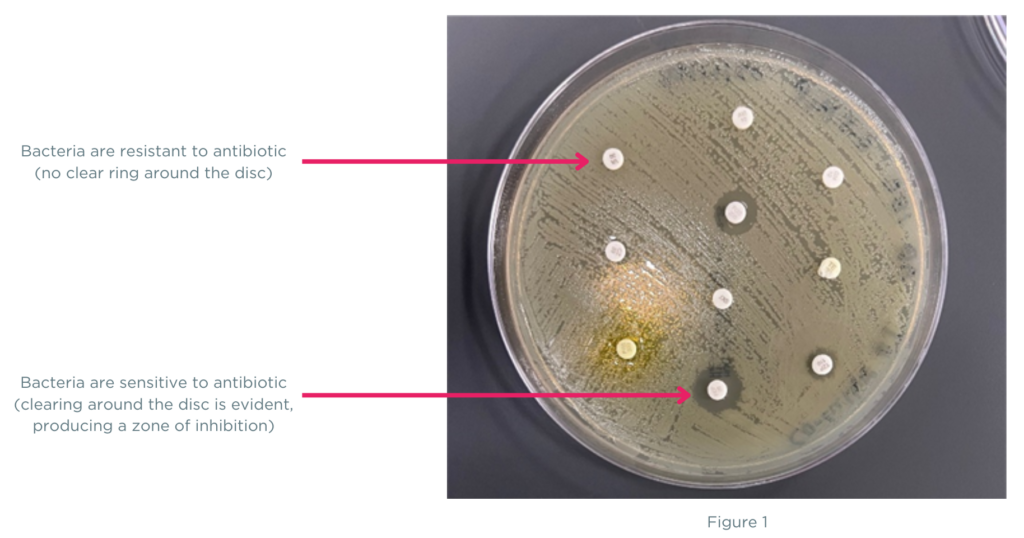
At first glance, the disk diffusion method appears to overcome the challenges of traditional sensitivity testing by providing phenotypic results, even for polymicrobial specimens. However, for polymicrobial cases with several organisms present in different amounts, the plated specimen will contain a combination of bacteria and may not be equally represented in the areas where the discs are placed (figure 2). The bacterial “lawn” grown on the agar plate is heterogenous for all the bacteria contained in the specimen, and the clear ring (zone of inhibition) may not represent all the bacteria found in the sample, just the bacteria species that grew in the area of the agar plate. These heterogeneous variations from disc diffusion can directly impact antibiotic sensitivity results that are the basis of patient antibiotic treatment plans.
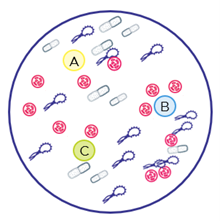 Figure 2
Figure 2
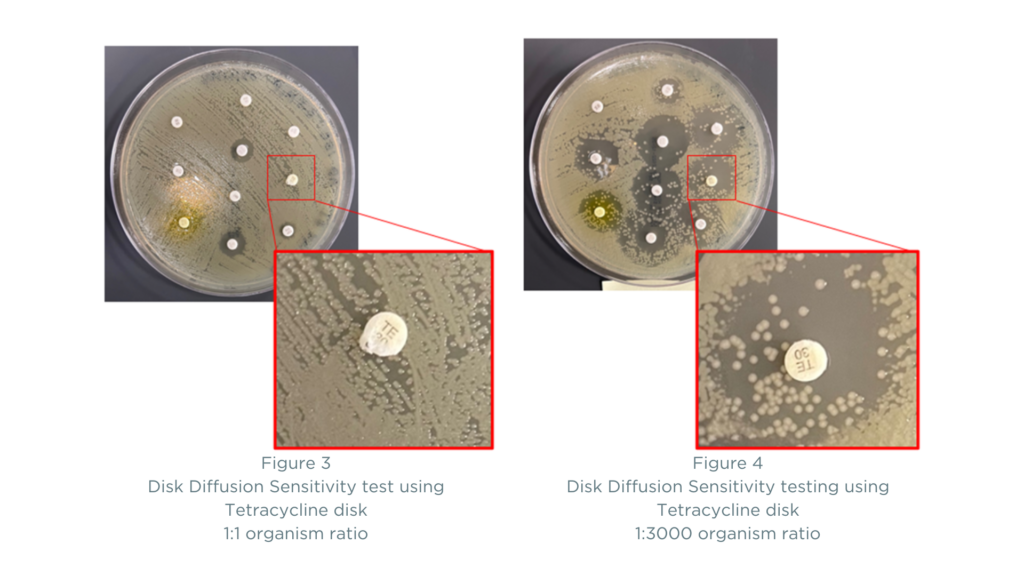
An example of bacteria heterogeneity and its effects on sensitivity results using disc diffusion can also be illustrated by the ratio of bacteria found in a specimen and its impact on the zone of inhibition. The images below demonstrate how antibiotic sensitivity results using the disk diffusion method can vary depending on the ratio of organisms being tested against an antibiotic disk. The two plates have different proportions of the organisms A. baumannii (known to be resistant to most antibiotics) and E. coli (known to be sensitive to most antibiotics). In Figure 3, the ratio of A. baumannii to E. coli on the plate is 1:1, and the results show resistance to Tetracycline. In Figure 4, the ratio of A. baumannii to E. coli is 1:3000 (which is a common finding in mixed infections). Here, you can see that the results become more difficult to interpret as many more antibiotics appear to be sensitive. Is the zone of inhibition clear? Or is the concentration of A. baumannii not high enough to clearly show resistance using the disk diffusion method? Reporting of antibiotic sensitivities can include results such as “intermediate” or “susceptible dose-dependent” in addition to “sensitive” and “resistant” findings, producing a need for interpretation of results and adding a level of scientific uncertainty.
An innovative technique called Pooled Antibiotic Susceptibility Testing (P-AST™) overcomes these variations associated with disk diffusion’s “lawn” of bacteria. When using the P-AST™ laboratory methodology, the ratio of organisms is distributed in such a way that the results are easily interpreted for each antibiotic (Figure 5).
Figure 5
The Guidance® UTI test provides an easy-to-read report that combines Multiplex Polymerase Chain Reaction (mPCR) for organism identification and resistance gene detection with P-AST™, allowing for more informed treatment decisions. The test has a large body of evidence, including five clinical studies with over 68,000 subjects and 10 peer-reviewed publications. Guidance® UTI is the only non-traditional UTI test that has demonstrated improved patient outcomes, reduced hospital utilization, and lower overall costs.
If you are ready for an innovative solution backed by clinical evidence when treating recurrent or complicated UTIs, watch a video explaining how the Guidance® UTI test report will provide more information for personalized therapy options.