A. urinae
Emery Haley, PhD, Scientific Writing Specialist
Aerococcus urinae
Clinical Summary
- A. urinae is a nitrite-negative, biofilm-forming, gram-positive microorganism.
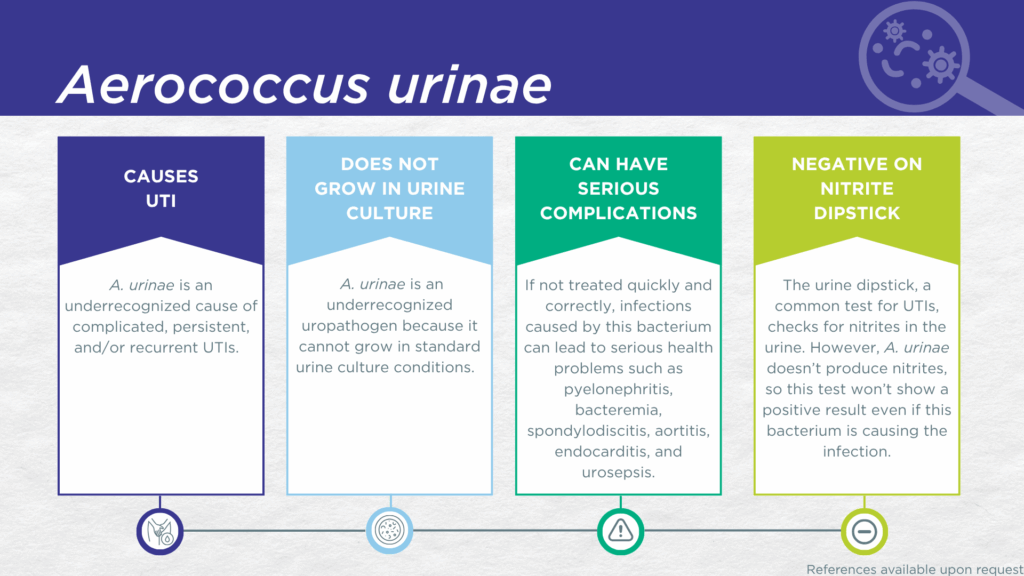
- A. urinae is fastidious and cannot grow in standard urine culture conditions.
- A. urinae is associated with complicated UTIs in children, older adults, and individuals with urinary tract abnormalities or multimorbidity.
- A. urinae preferentially infects kidney tissues.
- In symptomatic UTI patients, A. urinae:
- Is not a contaminant (is found in catheter-collected urine specimens).
- Is viable (can grow out in expanded culture conditions).
- Is pathogenic (associated with elevated urine biomarkers of infection).
- Reported severe complications of A. urinae UTI include pyelonephritis, bacteremia, spondylodiscitis, aortitis, endocarditis, and urosepsis.
Bacterial Characteristics
Gram-stain
Gram-positive
Morphology
Coccus
Growth Requirements
Fastidious (prefers blood-agar)
Facultative anaerobe
Nitrate Reduction
No
Urease
Negative
Biofilm Formation
Yes
Pathogenicity
Colonizer or Pathobiont
Clinical Relevance in UTI
A. urinae was first described in 1992 [1] and first sequenced in 2016 [2]. Genome sequencing of additional strain isolates revealed significant genetic diversity [3]and ultimately resulted in the recent splitting of these isolates into four species, including A. urinae and three novel species, A. tenax, A. mictus, and A. loyolae [4,5]. However, there is currently no known association between any of the genetically distinct species and particular clinical phenotypes.[6]
A. urinae is found in asymptomatic individuals as a resident of the human urogenital microbiome.[7] However, it has also been recognized for decades as a ‘rare’ pathogen isolated from human blood and urinary tract infections, predominantly in older adults,[8–15]children,[16–21] and individuals with urinary tract abnormalities or multimorbidity [7,8,15,16,18,22–24]. A. urinae has also been reported to participate in the formation of polymicrobial biofilms on indwelling catheters.[23]
A. urinae UTIs are likely significantly underdiagnosed due to the numerous challenges in the identification of A. urinae. Firstly, A. urinae lacks nitrate reductase activity, so screening strategies involving urinalysis for nitrite positivity will be false-negative.[25,26] Secondly, growing A. urinae in culture requires particular nutritional requirements (blood agar medium), which is not always included in clinical laboratories performing standard culture techniques for UTI diagnosis.[27] Thirdly, even when this organism does grow in culture, as a gram-positive coccus, it is frequently confused with Staphylococcus, Streptococcus, or Enterococcus species and may be dismissed as an irrelevant gram-positive “contaminant” organism from the urogenital microbiome.[15,27,28] Instead, A. urinae UTI is most often diagnosed by advanced proteomic techniques, such as Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS), or advanced molecular techniques, including polymerase chain reaction (PCR) and sequencing.[9,22,28–30]
A study using expanded quantitative urine culture (EQUC), a technique growing a larger urine volume with additional nutritional media, different atmospheric conditions, and longer incubations, found that the organism was viable. [31] In a study of older adult males and females with clinically suspected complicated UTI, A. urinae was detected in both midstream voided and in-and-out-catheter collected specimens indicating that it was truly present in the bladder, not simply a contaminant picked up during voiding.[30] Furthermore, elevated markers of immune system activation in the urinary tract have been measured from the same clinical urine specimens in which A. schaalii was detected, indicating that the presence of A. urinae was associated with an immune response to urinary tract infection.[32–34]
Critically, A. urinae demonstrates specific tropism for the kidney in mouse models of UTI.[6] Therefore, ascending infection with progression from cystitis to pyelonephritis is a significant risk. A. urinae in the kidney can readily cross into the bloodstream, resulting in bacteremia [22] that may progress to urosepsis [29]. A. urinae that reaches the bloodstream is also known to cause serious invasive infections including reports of spondylodiscitis,[35] aortitis,[36] endocarditis with aortic wall ulcer,[37] endocarditis of the aortic valve,[38] endocarditis with mycotic aneurysms,[39] and endocarditis requiring mitral valve replacement [40]. Indeed, strain matching of A. urinae isolated from the urine and from invasive disseminated infection sites confirms that the urinary tract is frequently the point of origin for such infection.[22,29,35]
Together, these findings demonstrate the value of detecting this organism and indicate that A. urinae should be seriously considered as a uropathogen when detected in any individual with UTI symptoms.
Treatment
Evidence of Efficacy (Checkmarks): Amoxicillin/Clavulanate, Ampicillin, Ampicillin/Sulbactam, Cefazolin, Ceftriaxone, Clindamycin, Doxycycline, Fosfomycin, Gentamicin, Linezolid, Meropenem, Nitrofurantoin, Piperacillin/Tazobactam, and Vancomycin.
- Aguirre, M.; Collins, M.D. Phylogenetic Analysis of Some Aerococcus-like Organisms from Urinary Tract Infections: Description of Aerococcus Urinae Sp. Nov. Microbiology+ 1992, 138, 401–405, doi:10.1099/00221287-138-2-401.
- Carkaci, D.; Dargis, R.; Nielsen, X.C.; Skovgaard, O.; Fuursted, K.; Christensen, J.J. Complete Genome Sequences of Aerococcus Christensenii CCUG 28831T, Aerococcus Sanguinicola CCUG 43001T, Aerococcus Urinae CCUG 36881T, Aerococcus Urinaeequi CCUG 28094T, Aerococcus Urinaehominis CCUG 42038 BT, and Aerococcus Viridans CCUG 4311T. Genome Announc. 2016, 4, e00302-16, doi:10.1128/genomea.00302-16.
- Carkaci, D.; Højholt, K.; Nielsen, X.C.; Dargis, R.; Rasmussen, S.; Skovgaard, O.; Fuursted, K.; Andersen, P.S.; Stegger, M.; Christensen, J.J. Genomic Characterization, Phylogenetic Analysis, and Identification of Virulence Factors in Aerococcus Sanguinicola and Aerococcus Urinae Strains Isolated from Infection Episodes. Microb. Pathog. 2017, 112, 327–340, doi:10.1016/j.micpath.2017.09.042.
- Choi, B.I.; Noronha, M.F.; Kaindl, J.; Wolfe, A.J. Complete Genome Sequences of Aerococcus Loyolae ATCC TSD-300 T , Aerococcus Mictus ATCC TSD-301 T , and Aerococcus Tenax ATCC TSD-302 T. Microbiol. Resour. Announc. 2024, e0015624, doi:10.1128/mra.00156-24.
- Choi, B.I.; Ene, A.; Du, J.; Johnson, G.; Putonti, C.; Schouw, C.H.; Dargis, R.; Senneby, E.; Christensen, J.J.; Wolfe, A.J. Taxonomic Considerations on Aerococcus Urinae with Proposal of Subdivision into Aerococcus Urinae, Aerococcus Tenax Sp. Nov., Aerococcus Mictus Sp. Nov., and Aerococcus Loyolae Sp. Nov. Int. J. Syst. Evol. Microbiol. 2023, 73, doi:10.1099/ijsem.0.006066.
- Gilbert, N.M.; Choi, B.; Du, J.; Collins, C.; Lewis, A.L.; Putonti, C.; Wolfe, A.J. A Mouse Model Displays Host and Bacterial Strain Differences in Aerococcus Urinae Urinary Tract Infection. Biol. Open 2021, 10, bio058931, doi:10.1242/bio.058931.
- Sierra-Hoffman, M.; Watkins, K.; Jinadatha, C.; Fader, R.; Carpenter, J.L. Clinical Significance of Aerococcus Urinae: A Retrospective Review. Diagn Micr Infec Dis 2005, 53, 289–292, doi:10.1016/j.diagmicrobio.2005.06.021.
- Balouch, B.; Munankami, S.; Acharya, A.; Shrestha, M.; Rijal, S.S. A Case Report of Aerococcus Urinae Urinary Tract Infection in an Elderly Male with Multimorbidity. Cureus 2022, 14, e26379, doi:10.7759/cureus.26379.
- Zabel, M.; Longfellow, L. Aerococcus Urinae Urinary Tract Infection in a Hospitalised Patient: An Increasingly Common Infection. BMJ Case Rep. 2023, 16, e257496, doi:10.1136/bcr-2023-257496.
- Yee, A.C.; Wong, T.T.J. Aero to the Heart: A Case of Aortic Valve Endocarditis Caused by Aerococcus Urinae in an Elderly Woman. IDCases 2023, 32, e01769, doi:10.1016/j.idcr.2023.e01769.
- Maraki, S.; Mavromanolaki, V.E.; Stafylaki, D. Aerococcus Urinae Urinary Tract Infections: A Case Series. Acta Microbiol. Immunol. Hung. 2024, 71, 324–328, doi:10.1556/030.2024.02419.
- Bradley, E.S.; Schell, B.; Ward, D.V.; Bucci, V.; Zeamer, A.; Haran, J.P. The Urinary Microbiome of Older Adults Residing in a Nursing Home Varies with Duration of Residence and Shows Increases in Potential Pathogens. Journals Gerontology Ser 2021, doi:10.1093/gerona/glab345.
- Lotte, L.; Durand, C.; Chevalier, A.; Gaudart, A.; Cheddadi, Y.; Ruimy, R.; Lotte, R. Acute Pyelonephritis with Bacteremia in an 89-Year-Old Woman Caused by Two Slow-Growing Bacteria: Aerococcus Urinae and Actinotignum Schaalii. Microorganisms 2023, 11, 2908, doi:10.3390/microorganisms11122908.
- Kakodkar, P.; Scott, J.; Tariq, J.; Du, L.; Wu, F.; Mehta, N.; Hamula, C. Emerging Pathogens Aerococcus Urinae and Aerococcus Sanguinicola from a Canadian Tertiary Care Hospital. Futur. Microbiol. 2024, 19, 1321–1332, doi:10.1080/17460913.2024.2383503.
- Higgins, A.; Garg, T. Aerococcus Urinae: An Emerging Cause of Urinary Tract Infection in Older Adults with Multimorbidity and Urologic Cancer. Urology Case Reports 2017, 13, 24–25, doi:10.1016/j.eucr.2017.03.022.
- Rast, D.; Evers, K.S.; Egli, A.; Rudin, C.; Goischke, A.; Ritz, N. Aerococcus Urinae — Significance of Detection in the Paediatric Urinary Tract: A Case Series. Eur. J. Pediatr. 2023, 182, 749–756, doi:10.1007/s00431-022-04730-2.
- Sato, E.; Iijima, H.; Shoji, K.; Ogimi, chikara; Ishiguro, A. 1696. Urinary Tract Infection Due to Aerococcus Urinae in Children. Open Forum Infect. Dis. 2023, 10, ofad500.1529, doi:10.1093/ofid/ofad500.1529.
- Sato, E.; Iijima, H.; Shoji, K.; Ishiguro, A.; Ogimi, C. Characteristics of Urinary Tract Infections in Children with Positive Urine Culture for Aerococcus Urinae. J. Infect. Chemother. 2024, 30, 1186–1189, doi:10.1016/j.jiac.2024.06.010.
- Dudani, R.; Qasem, A.; Udom, K. Aerococcus Urinae Infection Causing Malodorous Urine in a Child: A Case Report. Cureus 2024, 16, e55635, doi:10.7759/cureus.55635.
- Santhanam, H.; Muthukumarasamy, N.; Baidoun, M.; Behrle, N.M.; Loker, J.L. Aerococcus Urinae Infective Endocarditis in a Pediatric Structurally Normal Heart. Prog Pediatr Cardiol 2021, 101445, doi:10.1016/j.ppedcard.2021.101445.
- Wilson, J.R.; Bartley, E.E.; Anthony, H.D.; Brent, B.E.; Sapienza, D.A.; Chapman, T.E.; Dayton, A.D.; Milleret, R.J.; Frey, R.A.; Meyer, R.M. What Is Fishy in Asymptomatic Patients?: Co-Occurrence of Aerococcus Urinae Infection in Pediatric Patient with Phimosis. Acta Fac. Medicae Naissensis 2021, 38, 399–402, doi:10.5937/afmnai38-32051.
- Senneby, E.; Göransson, L.; Weiber, S.; Rasmussen, M. A Population-Based Study of Aerococcal Bacteraemia in the MALDI-TOF MS-Era. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 755–762, doi:10.1007/s10096-016-2594-z.
- Yu, Y.; Tsitrin, T.; Bekele, S.; Thovarai, V.; Torralba, M.G.; Singh, H.; Wolcott, R.; Doerfert, S.N.; Sizova, M.V.; Epstein, S.S.; et al. Aerococcus Urinae and Globicatella Sanguinis Persist in Polymicrobial Urethral Catheter Biofilms Examined in Longitudinal Profiles at the Proteomic Level. Biochem. Insights 2019, 12, 1178626419875089, doi:10.1177/1178626419875089.
- Shelton-Dodge, K.; Vetter, E.A.; Kohner, P.C.; Nyre, L.M.; Patel, R. Clinical Significance and Antimicrobial Susceptibilities of Aerococcus Sanguinicola and Aerococcus Urinae. Diagn Micr Infec Dis 2011, 70, 448–451, doi:10.1016/j.diagmicrobio.2010.09.001.
- BacDive | The Bacterial Diversity Metadatabase Available online: https://bacdive.dsmz.de/ (accessed on 11 February 2025).
- BioCyc Pathway/Genome Database Collection Available online: https://biocyc.org/ (accessed on 11 February 2025).
- Meletis, G.; Chatzidimitriou, D.; Tsingerlioti, F.; Chatzopoulou, F.; Tzimagiorgis, G. An Initially Unidentified Case of Urinary Tract Infection Due to Aerococcus Urinae. New Microbiol 2017, 40, 221–222.
- Cattoir, V.; Kobal, A.; Legrand, P. Aerococcus Urinae and Aerococcus Sanguinicola, Two Frequently Misidentified Uropathogens. Scand J Infect Dis 2010, 42, 775–780, doi:10.3109/00365548.2010.485576.
- Kim, J.; Hong, S.K.; Kim, M.; Yong, D.; Lee, K. MALDI-TOF-MS Fingerprinting Provides Evidence of Urosepsis Caused by Aerococcus Urinae. Infect. Chemother. 2017, 49, 227–229, doi:10.3947/ic.2017.49.3.227.
- Wang, D.; Haley, E.; Luke, N.; Mathur, M.; Festa, R.; Zhao, X.; Anderson, L.A.; Allison, J.L.; Stebbins, K.L.; Diaz, M.J.; et al. Emerging and Fastidious Uropathogens Were Detected by M-PCR with Similar Prevalence and Cell Density in Catheter and Midstream Voided Urine Indicating the Importance of These Microbes in Causing UTIs. Infect. Drug Resist. 2023, Volume 16, 7775–7795, doi:10.2147/idr.s429990.
- Festa, R.A.; Luke, N.; Mathur, M.; Parnell, L.; Wang, D.; Zhao, X.; Magallon, J.; Remedios-Chan, M.; Nguyen, J.; Cho, T.; et al. A Test Combining Multiplex-PCR with Pooled Antibiotic Susceptibility Testing Has High Correlation with Expanded Urine Culture for Detection of Live Bacteria in Urine Samples of Suspected UTI Patients. Diagn Microbiol Infect Dis 2023, 107, 116015, doi:10.1016/j.diagmicrobio.2023.116015.
- Haley, E.; Luke, N.; Mathur, M.; Festa, R.A.; Wang, J.; Jiang, Y.; Anderson, L.A.; Baunoch, D. The Prevalence and Association of Different Uropathogens Detected by M-PCR with Infection-Associated Urine Biomarkers in Urinary Tract Infections. Res. Rep. Urol. 2024, 16, 19–29, doi:10.2147/rru.s443361.
- Akhlaghpour, M.; Haley, E.; Parnell, L.; Luke, N.; Mathur, M.; Festa, R.A.; Percaccio, M.; Magallon, J.; Remedios-Chan, M.; Rosas, A.; et al. Urine Biomarkers Individually and as a Consensus Model Show High Sensitivity and Specificity for Detecting UTIs. BMC Infect Dis 2024, 24, 153, doi:10.1186/s12879-024-09044-2.
- Parnell, L.K.D.; Luke, N.; Mathur, M.; Festa, R.A.; Haley, E.; Wang, J.; Jiang, Y.; Anderson, L.; Baunoch, D. Elevated UTI Biomarkers in Symptomatic Patients with Urine Microbial Densities of 10,000 CFU/ML Indicate a Lower Threshold for Diagnosing UTIs. MDPI 2023, 13, 1–15, doi:10.3390/diagnostics13162688.
- Lyagoubi, A.; Souffi, C.; Baroiller, V.; Vallee, E. Aerococcus Urinae Spondylodiscitis: An Increasingly Described Localization. EJIFCC 2020, 31, 169–173.
- Chhibber, A.V.; Muttaiyah, S.; Hill, A.A.; Roberts, S.A. Aerococcus Urinae Aortitis: A Case Report. Open Forum Infect. Dis. 2019, 6, ofz453, doi:10.1093/ofid/ofz453.
- Ludhwani, D.; Li, J.; Huang, E.E.; Sikora, A.; Thomas, G. Aerococcus Urinae Aortic Valve Endocarditis with Kissing Aortic Wall Ulcer: A Case Report and Literature Review. Am. J. Case Rep. 2020, 21, e920974-1-e920974-6, doi:10.12659/ajcr.920974.
- Qureshi, N.K.; Patel, E. Aerococcus Urinae as the Causative Agent in Infective Endocarditis of the Aortic Valve in a Pediatric Patient. Pediatr. Infect. Dis. J. 2018, 37, 1065–1066, doi:10.1097/inf.0000000000001944.
- Sous, N.; Piwoz, J.A.; Baer, A.Z.; Bhavsar, S.M. Subacute Aerococcus Urinae Infective Endocarditis With Mycotic Aneurysms in a Pediatric Patient: Case Report and Literature Review. J. Pediatr. Infect. Dis. Soc. 2019, 8, 492–494, doi:10.1093/jpids/piz016.
- Yabes, J.M.; Perdikis, S.; Graham, D.B.; Markelz, A. A Rare Case of Aerococcus Urinae Infective Endocarditis in an Atypically Young Male: Case Report and Review of the Literature. BMC Infect. Dis. 2018, 18, 522, doi:10.1186/s12879-018-3414-0.
Dr. Emery Haley is a scientific writing specialist with over ten years of experience in translational cell and molecular biology. As both a former laboratory scientist and an experienced science communicator, Dr. Haley is passionate about making complex research clear, approachable, and relevant. Their work has been published in over 10 papers and focuses on bridging the gap between the lab and real-world patient care to help drive better health outcomes.